ABOUT US
Shenzhen Jitong Medical Registration Service Co., Ltd.
Shenzhen Jitong Medical Registration Service Co., Ltd. focuses on providing overseas compliance CRO services for medical device and in vitro diagnostic (IVD) enterprises. Relying on transnational self-operated companies established in multiple countries around the world and an experienced professional regulatory team, we create a one-stop full-life-cycle solution for customers, helping their products accelerate and successfully launch in the market, and comprehensively enhancing their core competitiveness in the international market.
I. Core Service System
(I) Regulatory Consulting and Registration Services
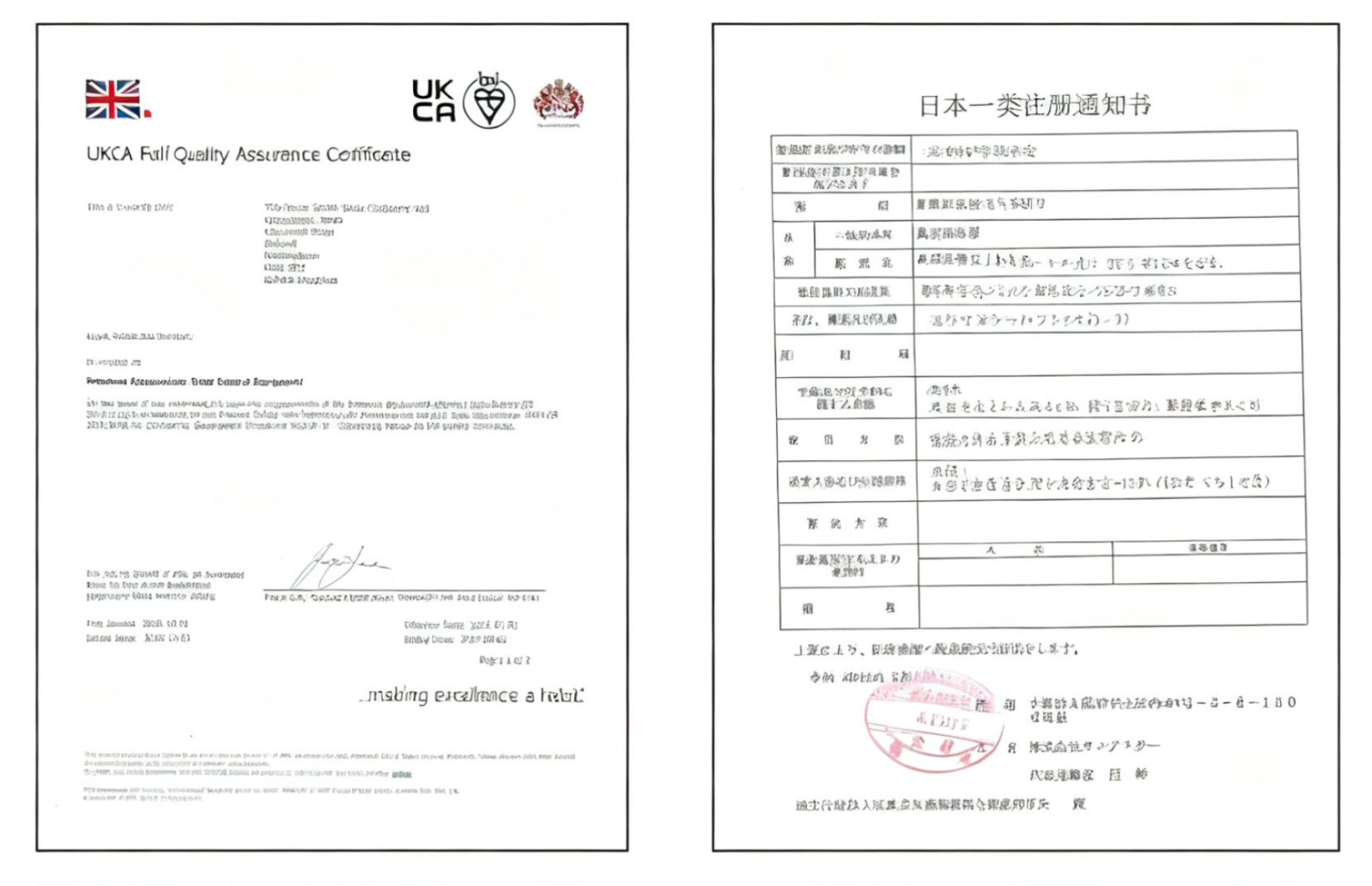
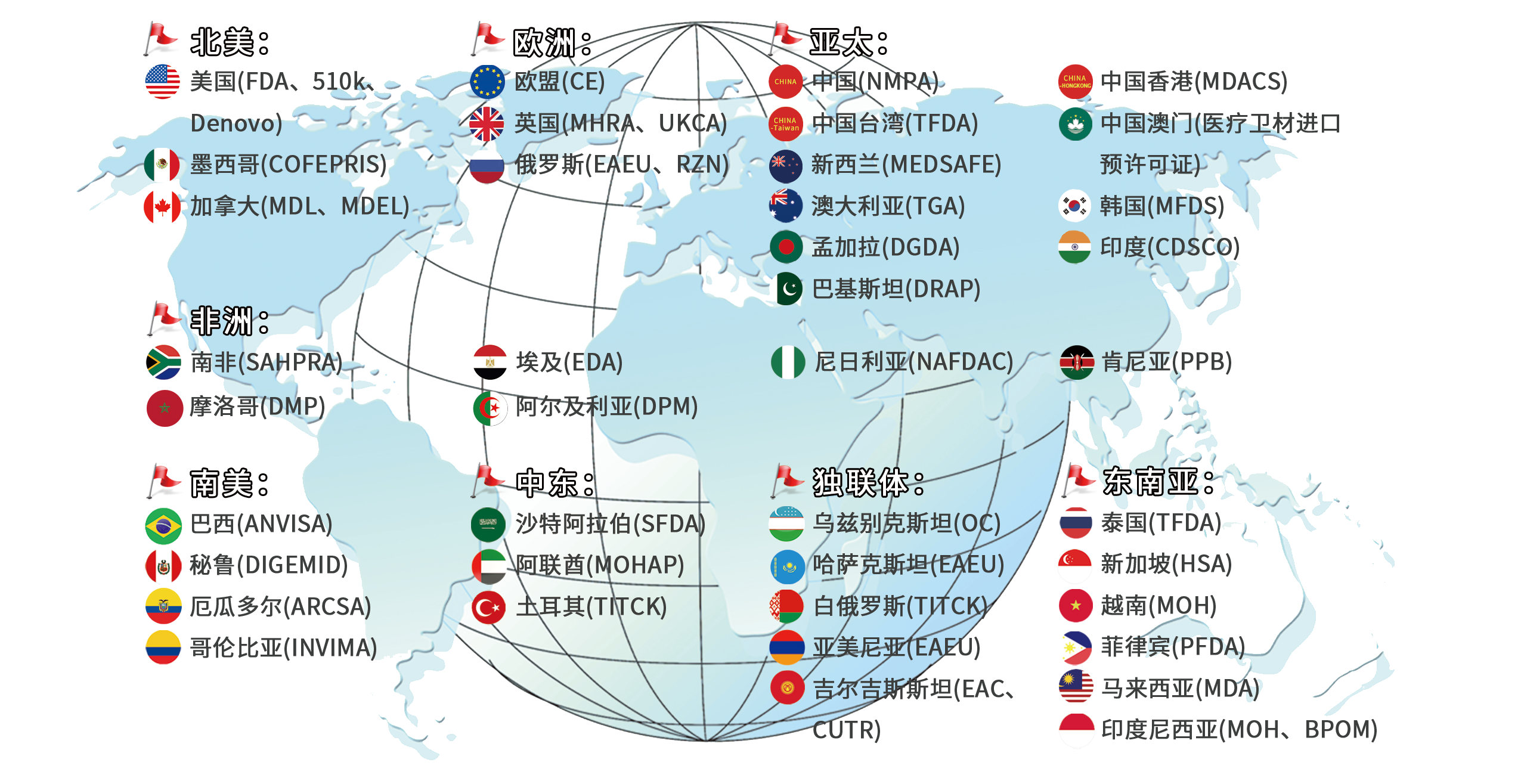
We provide regulatory consulting and registration support covering multiple regions and countries worldwide to help enterprises break through geographical compliance barriers, including:
Includes: clinical trial research, analytical performance studies, regulatory registration consultation, certification consultation, whitelist registration in various countries, product quality testing, system guidance, regulatory training, local authorized representatives, etc.













.png)
.png)
.png)
.png)